Take the stress out of autoclave testing and validation
Lee Morris explains the protocols around the testing and validation of autoclaves.
Autoclaves are essential in the day-to-day running of a dental practice to ensure reusable surgical instruments, handpieces and medical devices are made safe for use on patients and for handling by the dental team.
Infection control is often a task undertaken by dental nurses, so in an already busy schedule it is vital that autoclaves stay fully operational. Even minor problems can lead to downtime, causing health and safety implications and inconvenience to patients and staff.
Pay attention
Like any piece of dental equipment, autoclaves need regular attention to ensure they are functioning correctly and that infection control standards are being met.
All vacuum (B and S class) and non-vacuum (N class) autoclaves require routine testing and maintenance at pre-defined intervals that fall into three main categories: daily, weekly and annual tests. Daily and weekly tests can be carried out in-house by practice teams, while annual testing (servicing) and validation can only be carried out by a qualified engineer.
It is important to always follow the manufacturer’s guidance relating to testing, servicing and validation. The results for each autoclave should be recorded, preferably in a dedicated logbook, to provide a full audit trail of compliance with HTM 01-05, CQC, SDCEP and other relevant local guidelines.
Daily testing
For vacuum autoclaves, a daily Helix test measures the strength of steam penetration within the chamber. This demonstrates how effectively the steam in the autoclave penetrates hollow instruments such as handpieces, and therefore confirms the ability to achieve sterilisation.
For autoclaves not fitted with a data logger or printer a daily automatic control test using Class six indicator strips (also known as TST) measures time, steam and temperature within the autoclave.
Weekly tests
Vacuum (air leakage) tests need to be performed weekly. A vacuum test ensures the vacuum is operating correctly on B and S class autoclaves and to highlight any pump malfunctions and air leaks in the door seal or pipe fittings while ensuring that preset vacuum limits are reached.
Depending on the brand of autoclave, every 60 cycles (or as notified by the manufacturer) chamber cleaning tablets prevent the build-up of hard water deposits and limescale in the reservoir, chamber and tubing which if left untreated can prevent the machine from working properly and ultimately result in damage and unwanted repairs. Certain brands of autoclaves use tablets to clean the chamber only, they do not clean the reservoir or piping and this should always be checked with the manufacturer.
Annual service, PSI and validation
Annual servicing, PSI and validation can only be carried out by an engineer who has received training from a nationally accredited body. This is in order to safeguard the equipment and also to ensure compliance as required by regulatory bodies.
Autoclaves require an annual PSI (pressure system inspection) in accordance with Pressure System Safety Regulations (PSSR) 2000.
Some autoclaves require more frequent testing based on the number of sterilisation cycles rather than a specific timescale, so manufacturer’s guidance must always be followed. With any autoclave, annual validation is highly recommended and following any major repairs[i].
Clean inside and out
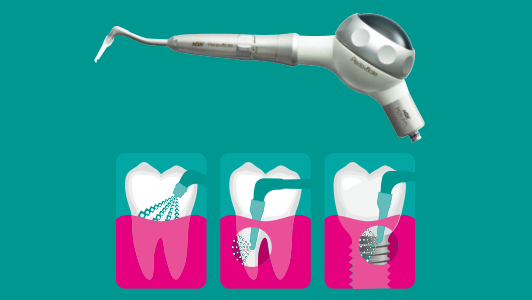
Prior to sterilisation it is essential that all instruments are free from debris, blood or organic residues before they are placed in the autoclave chamber. While it’s easy to see external contamination on a handpiece after surgery, the real problem is what’s happening on the inside.
Reaching the hidden internal surfaces and hollow channels (lumens) makes a handpiece difficult to clean and disinfect effectively. Any remaining protein residues such as blood can ‘bake’ onto internal surfaces during sterilisation, acting as a barrier to steam penetration and result in instruments not being processed correctly.
Successful cleaning of all external and internal surfaces of a handpiece is best achieved using a dedicated handpiece lubrication unit such as the NSK iCare. Such automated systems deliver the precise amount of lubrication to all parts of the mechanism, making it ready for subsequent sterilisation.
Prepare for the unexpected
Equipment breakdowns are not only frustrating, but can also mean unexpected repair bills and disruption to the running of the practice. Regular servicing reduces the risk of equipment breakdown and extends the lifespan of valuable equipment.
Flexible service plans offer a great way to optimise the life and performance of equipment, but it’s important to be clear about what is included and ensure that work is always carried out by a qualified engineer and that only genuine manufacturer parts are used in any repairs.
NSK offers a range of service plans specifically designed for NSK autoclaves that cover the cost of annual maintenance, testing and validation, service, parts and labour – even breakdowns and general wear and tear, something a warranty alone doesn’t cover. With fixed monthly direct debits over three and five-year plans plus free on-site operator training and technical software upgrades, this is a common sense, affordable option for any practice.
To find out more visit mynsk.co.uk/nsk-care/nsk-care-service/
[i] HTM 01-05 2013 Section 12.2