Safe, effective and efficient
David Gibson considers important factors in post-pandemic handpiece decontamination.
To date, the response to Covid-19 from UK dental regulatory bodies has mainly centred around the minimisation of aerosols in dental practice treatment rooms. ‘Dental standard operating procedure: Transition to recovery’, first published by the office of the chief dental officer in June and subsequently updated in October 2020, provided guidance on increasing ventilation, the application of fallow time and the importance of PPE, but contained little specific advice about instrument decontamination.
A year after practices reopened following the first lockdown, guidance regarding the reprocessing of dental instruments, especially handpieces, remains surprisingly deficient, despite the obvious ongoing risk of infection to both patients and practice staff if decontamination procedures are inadequate. In addition, maintaining patient flow through practices remains crucial to financial viability and delays caused by inefficient processes should not be tolerated.
The challenge
Dental practices have a duty of care to provide clinically effective and safe care to patients, as set out in the Health and Social Care Act 2012. Instrument decontamination is a vital component of this requirement, and with the latest figures (May 5, 2021) from the Office for National Statistics reporting more than 40 per cent of people testing positive for Covid-19 are asymptomatic, dental practices need to be sure that their decontamination protocols remain robust.
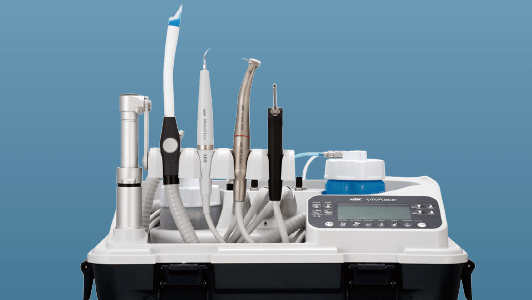
As part of our recommendations and as we navigate our way towards a post-pandemic environment, we will consider the journey handpieces take, from point of use in the surgery to the decontamination room and back again, ensuring the necessary steps have been taken to ensure they are safe for use on the next patient.
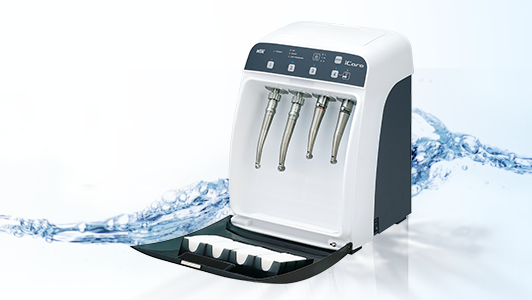
Cleaning hollow dental handpieces
In immediate response to the pandemic last summer, dental equipment manufacturers acted swiftly, adapting their products to minimise aerosol generation and providing practitioners with alternative non-AGP treatment options. For example, NSK’s Ti-Max Z95L and Z45L handpieces now have an anti-suck back mechanism that prevents blood and foreign matter entering the head, and a switch to turn off the air/water mix so that only water spray is used, thereby minimising aerosols.
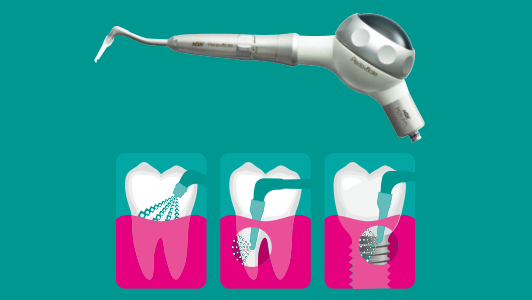
But cleaning the hidden internal surfaces and lumens of a handpiece remains a challenge. This essential cleaning is difficult to achieve using a manual process which can be inefficient and is difficult to reproduce consistently to a validated standard, potentially resulting in non-sterile instruments and even damage to the autoclave.
Once in the decontamination area all obvious signs of visible contamination on the external surface of the handpiece should be removed with a disposable wipe (do not use alcohol based products to clean as this can bind protein to the surface). Any soil can contain bacteria, viruses and spores which, if not removed, will act as a barrier to effective steam penetration, thereby preventing sterilisation. Once the external surfaces are clean, the internal components and lumens of the handpiece need to be cleaned and lubricated prior to sterilisation.
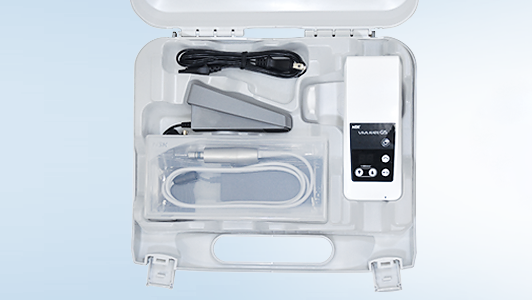
The ideal method of internal cleaning and lubrication is to use an automated unit, such as the NSK iCare. This equipment features technology specifically designed to quickly and effectively remove dirt and debris from the internal workings of handpieces and deliver the precise amount of lubricant in preparation for sterilisation. And, by connecting the iCare to a source of compressed air, any residual contaminants, solutions and lubricants will be automatically purged from the inside of the handpiece in a matter of seconds. This is arguably the most important facet of the whole handpiece decontamination process, which, if done correctly, will allow steam from the autoclave to penetrate inside these complex hollow instruments.
Vacuum sterilisation
Once handpieces have been cleaned and the internal working parts properly lubricated, they must be sterilised using the correct autoclave cycle.
One of the most complex challenges faced by decon teams is how to ensure effective sterilisation of their handpieces. This challenge is made all the more demanding in the face of less than clear guidance in this area, despite the fact that evidence from Winter (2016) clearly supports the fact that hollow instruments cannot be sterilised in a non-vacuum autoclave.
The solution to the conundrum of whether to use vacuum or non-vacuum cycles, as described in guidance, is relatively simple – minimise any possible risk by always using a vacuum autoclave. In most cases, this is achieved by the use of a built-in vacuum pump which sucks out all the air from the chamber and load (active air-removal) prior to introducing steam, as opposed to relying on gravity or downward displacement of air (passive air removal), as is the case when using a non-vacuum autoclave. The resultant vacuum created within the chamber and load ensures that the steam can fully penetrate and therefore sterilise instrument surfaces, both inside and out.
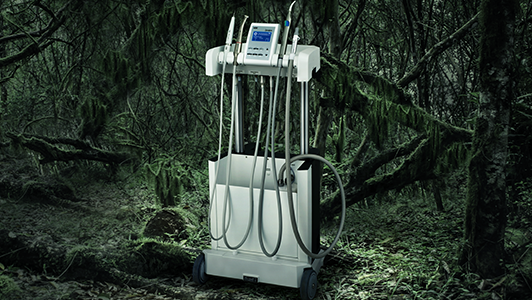
The copper-chambered NSK iMax and iClave vacuum autoclaves have been developed by NSK to effectively sterilise all instruments, especially dental handpieces. The use of a copper alloy chamber ensures superior thermal conductivity when compared to stainless steel counterparts, enabling the autoclaves to maintain a higher and more consistent temperature throughout the chamber, for the complete duration of the cycle. The adoption of this technology results in significantly faster cycles, meaning instruments can be sterilised and more importantly, fully dried, in less than 30 minutes, thus preventing bacterial re-contamination and corrosion. These are clear benefits for busy practices wanting to maintain optimal patient flow under current circumstances and reduce maintenance costs for these expensive instruments.
Safely back to chairside
Unless being sterilised in sealed pouches, as soon as any unpouched instruments are removed from the autoclave, they are at risk of recontamination and must therefore be protected until they are required again in the surgery.
The safest protocol is to pouch instruments (including handpieces) before sterilisation, where they remain in a sterile condition until they are opened in surgery. This process ensures that no airborne pathogens can reinfect the instrument before use. However, in the busy day-to-day routine of a practice, pouching before sterilisation can be impractical as it increases the cycle time of some autoclaves, slowing down the decontamination process.
Alternatively, instruments can be pouched immediately after sterilisation or sealed in a box for transport back to the surgery, ready for ‘immediate’ use or within that particular trearment session. If taking this approach, it is critical that instruments are completely dry before removal from the autoclave to avoid recontamination, which is more likely to occur if they remain damp or wet in the warmth of the surgery environment – ideal conditions in which micro-organisms can freely propagate. Instruments managed in this way cannot be considered sterile at point of use, only aseptically wrapped.